About Us
Guided by Purpose. Backed by Experience.
We believe that proven performance shouldn’t come at a premium. That’s why we deliver clinically backed, surgeon-trusted implants that align with both clinical needs and economic realities—helping surgeons restore mobility, reduce recovery time, and get their patients back to full functioning.
Whether you’re performing a complex fusion or a routine procedure, CA Medical is your partner in precision, performance, and patient care
Why Choose CA Medical
FDA-cleared solutions
Anatomically contoured implant designs
Surgeon-centered support

Our Commitment
Industry insight you can COUNT ON
Years of Industry Experience


Case Study
The Difference
One of the unique aspects of PEEK-OPTIMA HA Enhanced is that it addresses the entire interbody environment. HA crystals in PEEK-OPTIMA HA Enhanced are fully integrated, not coated, into the PEEK-OPTIMA matrix, making it available on all surfaces of a finished device. Consequently, both inter-cage and outer-cage graft material are exposed to hydroxyapatite, resulting in enhanced osteoconductivity.
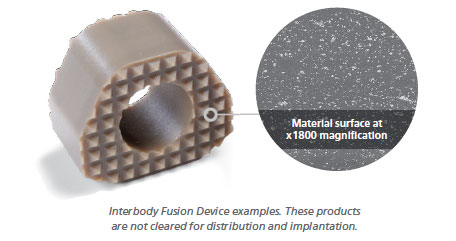
Early Clinical Experience
9-Patient Case Series (males and females aged 39-76 with varying levels of health, pre-existing diseases or conditions and previous surgeries.)
All nine patients underwent a one- or two-level lumbar fusion utilizing the same Interbody Devices. All devices were made with PEEK-OPTIMA HA Enhanced Polymer.
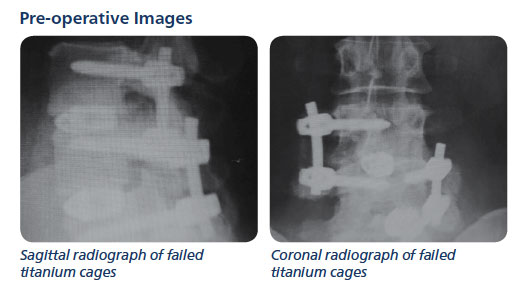
Example Patient 9, image above
Lumbar Fusion Case Study – A female who had two previous, two-level MIS procedures with pedicle screw constructs, titanium cages, infuse bone morphogenetic (BMP) and dbx putty. Both failed.
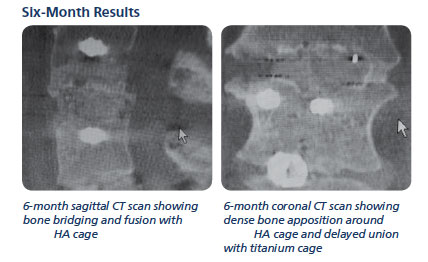
Results
Six-month post-op follow up showed solid fusion at 17 of the 17 levels.
Improved neurologic function in all 8 patients
Arm pain resolved in all 8 patients
Neck pain resolved in 5 of 8 patients
Neck pain improved in 7 of 8 patients
The patient reported no further leg pain and the case was deemed successful.
Let’s Make Your Job
Easier.
You don’t need more vendors—you need a partner.
