Products
PEEK OPTIMA- HA Spinal Implants by CA Medical
Optimized for Fusion. Designed for Confidence.

What is PEEK OPTIMA-HA
Enhanced Osseointegration
Radiolucency with Diagnostic Clarity
Bone-Matched Biomechanics
Exceptional Biocompatibility
Cervical Cage

PEEK-HA cervical cage is an interbody spinal implant to provide a stable surface for bone to fuse to and ensures a secure lock between implant and vertebrae. Available in both parallel and lordotic as well as a variety of sizes it allows surgeons to choose the best option for patients.
Key Features:
Optimized for Fusion
Radiolucent with Marker
Versatile Sizing
Ideal Applications:
Used to treat a variety of cervical spine disorders, such as degenerative disc disease, herniated discs, spinal stenosis, and instability.
Aids in the restoration of disc height, relieves nerve pressure, stabilises the spine, and promotes vertebral fusion.
Promotes bone development and fusion between adjacent vertebrae,to aid in the healing process.
PLIF Cage

Posterior Lumbar Interbody Fusion Cages (PLIF) stabilize and fuse vertebrae in the lumbar spine designed to restore disc height, promote fusion, and reduce pressure on nerves. Available in both parallel and lordotic as well as a variety of sizes to allow surgeons to choose the best option for patients.
Key Features:
Restores Disc Height
Versatile Sizing
Reduces Nerve Compression
Ideal Applications:
Used to treat a variety of lumbar spinal disorders: degenerative disc disease, herniated discs, spinal stenosis, and instability.
Aids in the restoration of disc height, relieves nerve pressure, stabilises the spine, and promotes vertebral fusion.
Promotes bone development and fusion between adjacent vertebrae,to aid in the healing process.
ALIF Cage
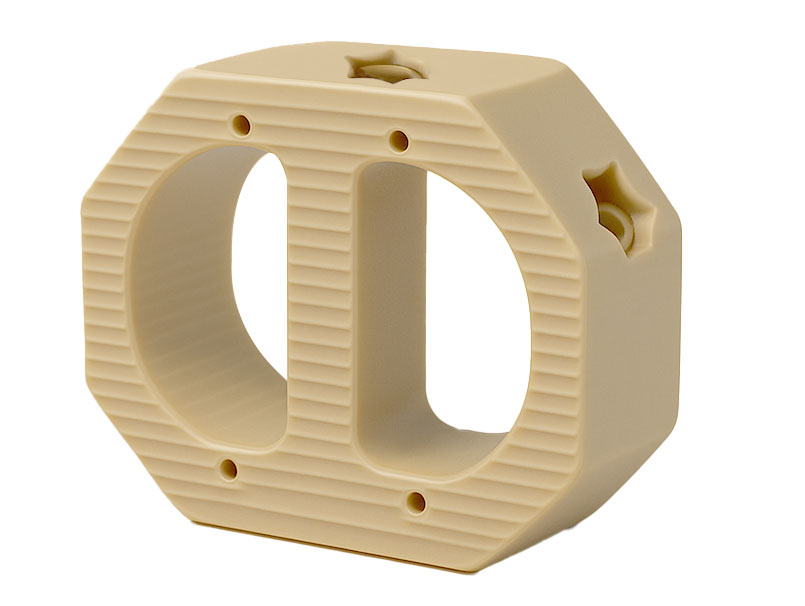
Key Features:
Anterior Approach Access
Wide Range of Sizes
Enhanced Stability
Ideal Applications:
Used to treat a variety of lumbar spinal disorders: degenerative disc disease, herniated discs, spinal stenosis, and instability.
Aids in the restoration of disc height, relieves nerve pressure, stabilises the spine, and promotes vertebral fusion.
Promotes bone development and fusion between adjacent vertebrae,to aid in the healing process.
TLIF Cage
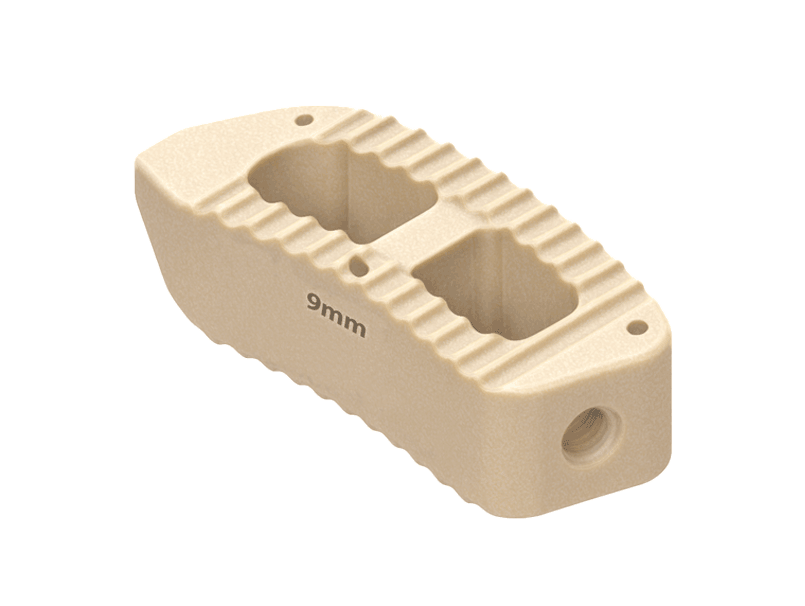
Key Features:
Restore Disc Height
Wide Range of Sizes
Relieves Nerve Pressure
Ideal Applications:
Used to treat a variety of Lumbar spinal disorders, such as degenerative disc disease, herniated discs, spinal stenosis, and instability through an anterior fusion procedure with high fusion rates.
Aids in the restoration of disc height, relieves nerve pressure, stabilises the spine, and promotes vertebral fusion.
Promotes bone development and fusion between adjacent vertebrae, to aid in the healing process.
Case Study
The Difference
One of the unique aspects of PEEK-OPTIMA HA Enhanced is that it addresses the entire interbody environment. HA crystals in PEEK-OPTIMA HA Enhanced are fully integrated, not coated, into the PEEK-OPTIMA matrix, making it available on all surfaces of a finished device. Consequently, both inter-cage and outer-cage graft material are exposed to hydroxyapatite, resulting in enhanced osteoconductivity.
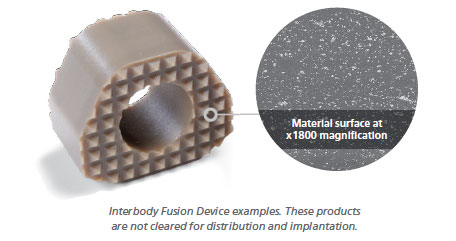
Early Clinical Experience
9-Patient Case Series (males and females aged 39-76 with varying levels of health, pre-existing diseases or conditions and previous surgeries.)
All nine patients underwent a one- or two-level lumbar fusion utilizing the same Interbody Devices. All devices were made with PEEK-OPTIMA HA Enhanced Polymer.
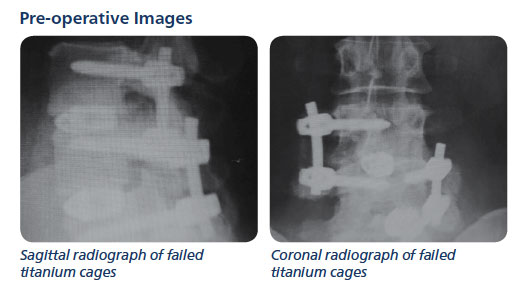
Example Patient 9, image above
Lumbar Fusion Case Study – A female who had two previous, two-level MIS procedures with pedicle screw constructs, titanium cages, infuse bone morphogenetic (BMP) and dbx putty. Both failed.
Results
Six-month post-op follow up showed solid fusion at 17 of the 17 levels.
Improved neurologic function in all 8 patients
Arm pain resolved in all 8 patients
Neck pain resolved in 5 of 8 patients
Neck pain improved in 7 of 8 patients
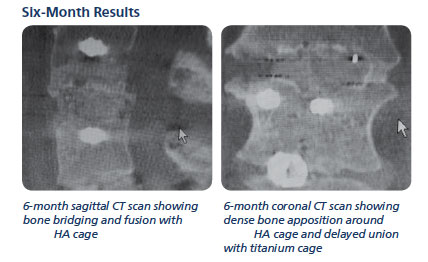
The patient reported no further leg pain and the case was deemed successful.
Stronger fusion.
Smarter materials.
Better outcomes.
Partner with CA Medical and experience the future of spinal implant technology.
