Restoring Motion.
Rebuilding Lives.
Advanced Spinal Implants That Deliver PEEK-HA Innovation Without Compromising Care or Cost.
A Partner That Has Your Back (Literally)
CA Medical delivers FDA-cleared, clinically backed spinal implants for hospitals and surgical distributors who want better outcomes—without the bloated costs.
With over 30 years of industry experience behind us, we know what it takes to serve both the OR and the bottom line. Let’s make your job easier. More About CA Medical
%
Increase in Spinal Procedures Over the Last 5 Years
73% of those being lumbar and cervical fusions
20+ Years of Proven Clinical History
Our PEEK Optima polymers are established as being safe, long-term implantable polymers.
%
Increase in Annual Lumbar Fusion Procedures
With a compounded annual growth rate or CAGR of 4.7%
SUPERIOR SPINE IMPLANTS
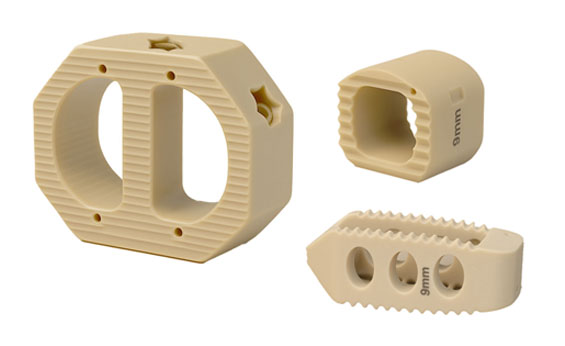
CA Medical Interbody Fusion Cages
CA Medical’s interbody fusion cages are engineered to meet the demands of both cervical and lumbar spine surgery—delivering structural integrity, anatomical compatibility, and superior imaging clarity. Made from advanced PEEK-HA, Our cages support optimal bone fusion while preserving radiolucency for post-op visualization.
With a wide range of sizes, angles, and footprints, our implants empower surgeons with the flexibility and confidence needed to provide their patients optimal care and faster recovery.
Case Study
The Difference
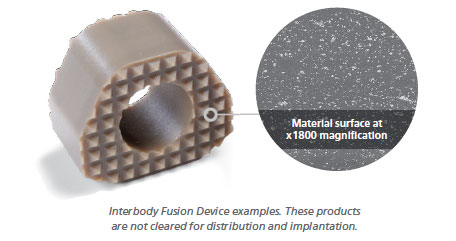
One of the unique aspects of PEEK-OPTIMA HA Enhanced is that it addresses the entire interbody environment. HA crystals in PEEK-OPTIMA HA Enhanced are fully integrated, not coated, into the PEEK-OPTIMA matrix, making it available on all surfaces of a finished device. Consequently, both inter-cage and outer-cage graft material are exposed to hydroxyapatite, resulting in enhanced osteoconductivity.
Early Clinical Experience
9-Patient Case Series (males and females aged 39-76 with varying levels of health, pre-existing diseases or conditions and previous surgeries.)
All nine patients underwent a one- or two-level lumbar fusion utilizing the same Interbody Devices. All devices were made with PEEK-OPTIMA HA Enhanced Polymer.
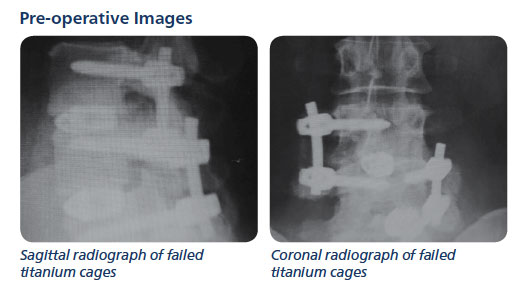
Example Patient 9, image above
Lumbar Fusion Case Study – A female who had two previous, two-level MIS procedures with pedicle screw constructs, titanium cages, infuse bone morphogenetic (BMP) and dbx putty. Both failed.
Results
Six-month post-op follow up showed solid fusion at 17 of the 17 levels.
Improved neurologic function in all 8 patients
Arm pain resolved in all 8 patients
Neck pain resolved in 5 of 8 patients
Neck pain improved in 7 of 8 patients
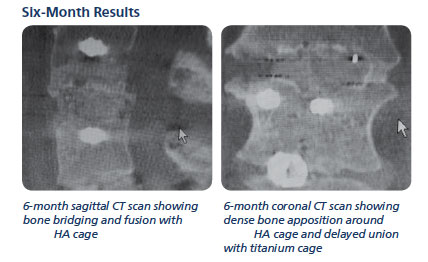
The patient reported no further leg pain and the case was deemed successful.
Let’s Make Your Job
Easier.
You don’t need more vendors—you need a partner.
